are electronic signatures hipaa compliant





Are Electronic Signatures HIPAA Compliant? Understanding Legal and Regional Requirements
In today’s fast-paced digital world, electronic signatures (e-signatures) have become the go-to solution for streamlining paperwork, improving workflow efficiency, and enhancing document security. But for industries like healthcare where sensitive data is involved, such as medical records and prescription confirmations, questions surrounding regulation compliance are critical—especially with laws like HIPAA (Health Insurance Portability and Accountability Act).
This article aims to answer a pressing question for healthcare providers, IT administrators, and compliance managers: Are electronic signatures HIPAA compliant? We’ll also explore regional nuances in markets like Hong Kong and Southeast Asia where healthcare providers must abide by both local and international data privacy laws.
Understanding HIPAA and Electronic Signatures
HIPAA is a federal law in the United States enacted in 1996 to protect sensitive patient health information from being disclosed without the patient’s consent or knowledge. One component of HIPAA is the HIPAA Security Rule, which sets standards for safeguarding electronic protected health information (ePHI).
To be HIPAA-compliant, an electronic signature solution must meet specific security requirements:
- Employ authentication of the signer’s identity
- Ensure non-repudiation to prevent denial of signature validity
- Maintain integrity of the signed data
- Use audit trails that log who signed the document and when
Note that HIPAA does not specifically endorse or reject the use of electronic signatures, but it mandates that healthcare providers and associates implement measures that guarantee data security and access controls.
What Makes an e-Signature HIPAA Compliant?
For an electronic signature to be considered HIPAA compliant, the platform must offer technical safeguards equal to those outlined in the HIPAA Security Rule. Here are the key features that a compliant solution should have:
1. Robust Access Controls
Only authorized users should be able to access and sign documents containing ePHI. E-signature platforms must support controls such as multi-factor authentication (MFA), role-based access, and user-level permissions.
2. Strong Audit Trails
The platform must create and maintain detailed records of all activities associated with a document, including timestamps for every access event, signature, and change.
3. Data Encryption
Healthcare documents must be encrypted both in transit and at rest to prevent unauthorized disclosure.
4. Signed Business Associate Agreement (BAA)
A HIPAA-covered entity must sign a BAA with the e-signature provider to hold them accountable for HIPAA compliance. Without this agreement, the provider cannot be trusted to handle protected data lawfully.

Are Popular e-Signature Platforms HIPAA-Compliant?
Many widely-used platforms such as DocuSign, Adobe Sign, and HelloSign offer HIPAA-compliant solutions—provided that users sign a Business Associate Agreement (BAA) with the provider.
However, compliance doesn’t just hinge on the platform itself. How organizations implement and use the solution is just as crucial. Misuse, such as granting access to unauthorized personnel or failing to monitor access logs, can still lead to HIPAA violations.
Legal Considerations in Asia: What About Hong Kong and Southeast Asia?
While HIPAA applies to the United States, healthcare organizations operating in Hong Kong, Singapore, Malaysia, and other parts of Southeast Asia must consider local data privacy laws too.
In Hong Kong:
Covered under the Personal Data (Privacy) Ordinance (PDPO), healthcare providers must ensure that sensitive health-related data is only used with patient consent and is adequately protected. While PDPO does not explicitly call out electronic signatures, any solution must meet data privacy requirements—including identity authentication and secure storage.
In Singapore:
Singapore’s Personal Data Protection Act (PDPA) requires compliance with consent, purpose limitation, and data protection obligations. E-signature platforms should support tamper-proof document storage and record management to be valid and secure.
In Malaysia:
The Digital Signature Act 1997 and PDPA regulate digital and electronic signatures. For legality and enforceability, the solutions must integrate with national standards like MyKad for identity or fulfill requirements set by licensed certification authorities.

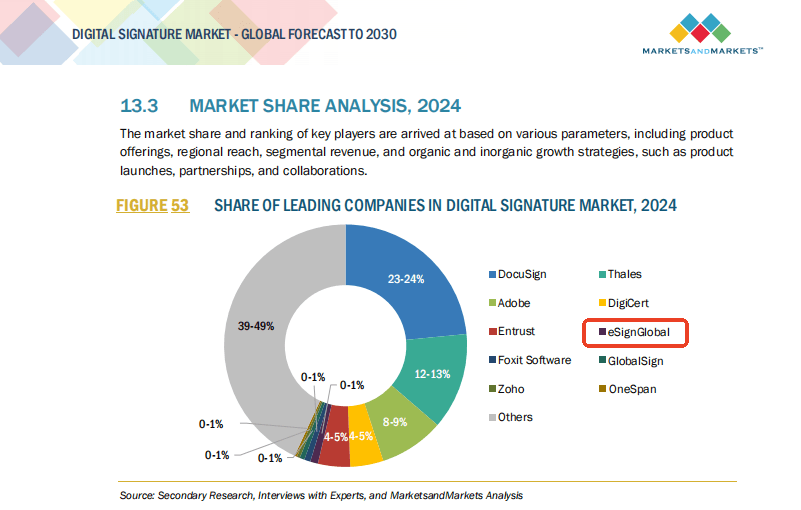
eSignGlobal: A Regional HIPAA-Compatible and PDPA-Compliant Alternative
For organizations operating across borders, ensuring compliance with both HIPAA and local privacy regulations is a challenge. This is where eSignGlobal shines as a solution tailored to regional needs.
Unlike global providers that focus heavily on US markets, eSignGlobal offers features aligned with Asian legal frameworks including PDPO, PDPA, and more—without compromising HIPAA compatibility for US-facing partners.
Why Choose eSignGlobal?
- HIPAA-compliant infrastructure with end-to-end encryption
- Automatic audit trails with secure timestamping
- Regional data storage options to meet local data residency laws
- Compliance with BAA, PDPA, PDPO, and digital signature laws across jurisdictions
- Chinese and English interfaces for local users
Best Practices: How to Ensure HIPAA Compliance with E-signatures
Here are five actionable tips to ensure your e-signature implementation remains legally sound:
- Always Sign a BAA with your provider before transmitting any ePHI.
- Implement access controls such as multi-factor authentication.
- Train employees on correct usage and handling of digitally-signed data.
- Choose platforms that allow setting up permissions, roles, and document expirations.
- Conduct periodic audits to ensure policy adherence and catch any potential violations.
Final Thoughts
To answer the question: Yes, electronic signatures can be HIPAA compliant—but only if deployed properly, with a platform that meets all technical and regulatory requirements.
Healthcare organizations in Hong Kong and Southeast Asia must look beyond HIPAA and examine local ordinances to ensure full legal alignment. Selecting an e-signature solution with regional legal knowledge and technical capability is no longer optional—it is a necessity.
That’s why for Asia-based professionals, Docusign alternatives such as eSignGlobal offer the best blend of HIPAA compliance, regional integration, and localized support.

 Only business email allowed
Only business email allowed


