docusign 21 cfr part 11 pricing





Understanding 21 CFR Part 11 and Its Relevance to Electronic Signatures
In the regulated industries like pharmaceuticals, biotechnology, and medical devices, compliance with 21 CFR Part 11 is essential for electronic records and signatures. This FDA regulation ensures the integrity, authenticity, and reliability of electronic documents, mandating features such as audit trails, electronic signatures with identity verification, and secure record controls. Businesses in these sectors often turn to e-signature platforms like DocuSign to meet these standards while streamlining workflows. However, the cost of achieving full compliance can be a significant consideration, especially when integrating advanced security and reporting tools.

DocuSign’s Approach to 21 CFR Part 11 Compliance
DocuSign positions itself as a leader in compliant e-signature solutions, offering features that align with 21 CFR Part 11 requirements. These include tamper-evident audit trails that record every action on a document, validation of electronic signatures through multi-factor authentication, and controls to prevent unauthorized access or alterations. For instance, DocuSign’s platform supports digital certificates and knowledge-based authentication to verify signer identity, ensuring records are attributable and non-repudiable.
To access these compliance capabilities, users typically need to opt for higher-tier plans rather than basic subscriptions. The Personal and Standard plans provide foundational e-signing but lack the robust governance and reporting needed for Part 11. Instead, DocuSign’s Business Pro and especially the Advanced Solutions (Enterprise) tiers incorporate Part 11-specific tools like advanced audit reporting, data encryption at rest and in transit, and integration with enterprise identity management systems.
Breaking Down DocuSign 21 CFR Part 11 Pricing
Pricing for DocuSign’s 21 CFR Part 11 compliant features is not straightforward, as it ties into their subscription model with add-ons and custom enterprise agreements. At its core, DocuSign structures costs around user seats, envelope volumes (each envelope represents a document or signing process), and automation limits. For basic eSignature plans without full compliance:
-
Personal Plan: $120 per year ($10/month) for one user and up to 5 envelopes monthly. This is insufficient for regulated environments due to limited audit and security depth.
-
Standard Plan: $300 per user per year ($25/month), supporting team collaboration and up to 100 envelopes per user annually. It includes basic reminders and templates but falls short on Part 11’s audit trail granularity.
-
Business Pro Plan: $480 per user per year ($40/month), adding web forms, conditional logic, and bulk send capabilities. While it enhances workflow efficiency, full Part 11 compliance requires supplementary configurations, potentially increasing costs through add-ons.
The real focus for 21 CFR Part 11 shifts to DocuSign’s Advanced Solutions and Enterprise plans, which are not publicly priced and demand contacting sales for customization. These plans bundle SSO (Single Sign-On), advanced governance, premium audit logs, and dedicated compliance support—essential for FDA validation. Based on industry reports and verified overviews from 2024-2025, enterprise pricing starts around $10,000-$50,000 annually for small teams (10-50 users), scaling with factors like envelope volume (often 100+ per user/year), API integrations, and regional compliance needs.
Add-ons further inflate costs for Part 11 adherence:
- Identity Verification (IDV): Metered at extra per-use fees (e.g., $1-5 per verification), including biometric checks and SMS authentication to meet signer validation rules.
- SMS/WhatsApp Delivery: Per-message charges, varying by region (e.g., $0.10-$0.50 per SMS), useful for timely notifications in compliant workflows.
- API Access for Automation: Developer plans like Intermediate ($3,600/year for ~100 envelopes/month) or Advanced ($5,760/year) enable programmatic compliance checks, but quotas cap automation sends (e.g., ~10/month per user).
Envelope limits are a hidden cost driver: Even “unlimited” plans cap automation (bulk sends, PowerForms) at ~100 per user/year, leading to overage fees of $0.50-$2 per excess envelope. For a mid-sized pharma firm with 20 users handling 500 compliant documents annually, total costs could exceed $15,000-$30,000, excluding implementation consulting (often $5,000+). Enterprise customizations for Part 11 might add 20-50% premiums for features like data residency and enhanced reporting.
From a business perspective, this tiered, usage-based model ensures scalability but can surprise users with opaque total ownership costs. Public documentation emphasizes annual billing discounts (up to 20% off monthly rates), yet without a clear quote, budgeting for Part 11 compliance remains challenging. In regulated sectors, where non-compliance risks fines up to $250,000 per violation, these investments are justifiable, but smaller organizations may find the entry barrier high.
Challenges in DocuSign’s Pricing and Global Service Delivery
While DocuSign excels in core compliance, its pricing strategy draws criticism for opacity and high costs. Base plans seem affordable, but layering on Part 11 features—through enterprise negotiations—often results in premiums that double or triple expectations. Overage fees for envelopes or verifications accumulate quickly in high-volume scenarios, and API quotas force upgrades without proportional value for all users.
Service inconsistencies compound these issues, particularly in long-tail regions like APAC. Cross-border latency slows document loading and signing, critical for time-sensitive pharma trials. Compliance tools may require extra governance add-ons for local data residency, inflating costs by 15-30%. In China and Southeast Asia, limited native ID verification methods and higher support fees create friction, as DocuSign’s global infrastructure prioritizes North America and Europe. This can lead to delayed audits or integration hurdles, frustrating multinational teams reliant on seamless Part 11 workflows.
Comparing DocuSign, Adobe Sign, and eSignGlobal for Compliance Needs
DocuSign sets the benchmark for 21 CFR Part 11 with its mature ecosystem, but alternatives like Adobe Sign and eSignGlobal offer competitive options, especially for cost-conscious or regionally focused businesses.
Adobe Sign, integrated with Adobe’s Document Cloud, supports Part 11 through features like audit trails, e-sign validation, and API extensibility. Pricing mirrors DocuSign’s structure: Individual plans at $10/month, Business at $25/user/month, and Enterprise custom (often $20,000+ annually for compliance setups). It shines in creative industries with PDF editing but faces similar add-on fees for IDV and SMS. However, Adobe’s withdrawal from certain markets, like China, limits its appeal for APAC operations.
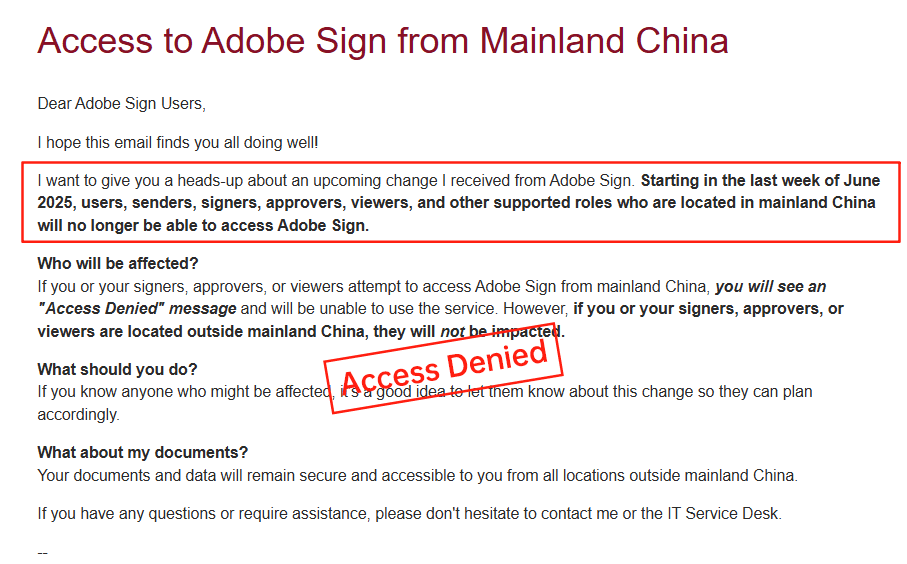
eSignGlobal, a rising player optimized for APAC and emerging markets, provides Part 11-compliant e-signatures with region-native compliance. Its pricing is more transparent and flexible: Core plans start at $15/user/month for standard features, with enterprise bundles around $8,000-$20,000/year including unlimited envelopes in compliant modes. It emphasizes low-latency delivery, local data centers, and integrated biometric verification without heavy add-ons, making it suitable for cross-border pharma workflows.
Here’s a neutral comparison across key aspects:
| Aspect | DocuSign | Adobe Sign | eSignGlobal |
|---|---|---|---|
| 21 CFR Part 11 Support | Robust (audit trails, SSO, advanced reporting in Enterprise) | Strong (integrated with Acrobat, validation tools) | Compliant (biometrics, local audit logs, FDA-aligned) |
| Base Pricing (Annual, per User) | $300-$480 (Standard/Pro); Custom Enterprise | $240-$360 (Business); Custom Enterprise | $180+ (Flexible tiers) |
| Envelope Limits | ~100/user/year; Overages $0.50+ | ~100/user/year; Similar overages | Unlimited in compliant plans |
| Add-On Costs (IDV/SMS) | Metered ($1-5/verification; $0.10+/message) | Metered, bundled in Enterprise | Included or low flat fees |
| APAC Performance | Latency issues; Extra compliance fees | Limited availability (e.g., China exit) | Optimized (local servers, fast delivery) |
| Transparency | Low (Custom quotes dominant) | Moderate (Bundled with Adobe ecosystem) | High (Clear public tiers) |
| Best For | Global enterprises with high budgets | PDF-heavy workflows | APAC/SEA regulated industries |
This table highlights trade-offs: DocuSign and Adobe offer ecosystem depth but at higher, less predictable costs, while eSignGlobal prioritizes affordability and regional efficiency without sacrificing core compliance.
Recommendations for DocuSign Alternatives in Regulated Markets
For businesses seeking 21 CFR Part 11 compliance without DocuSign’s premium pricing or global service gaps, eSignGlobal emerges as a strong regional alternative. Its focus on APAC optimization, transparent costs, and native compliance makes it ideal for pharma and biotech firms expanding in Asia, providing a balanced path to regulatory adherence. Ultimately, the choice depends on scale and geography—evaluating demos from multiple providers ensures the best fit.
FAQs

 Only business email allowed
Only business email allowed


