DocuSign CLM for Pharma: Ensuring strict regulatory compliance





Navigating Regulatory Compliance in Pharmaceutical Contract Management
The pharmaceutical industry operates under some of the most stringent regulatory frameworks globally, where even minor lapses in contract handling can lead to severe penalties, delayed product launches, or reputational damage. Electronic signature and contract lifecycle management (CLM) tools have become essential for streamlining processes while ensuring adherence to standards like FDA 21 CFR Part 11, EU eIDAS, and HIPAA. In this context, DocuSign’s CLM offerings stand out for their focus on pharma-specific compliance needs.

The Imperative of Compliance in Pharma Contracts
Understanding Key Regulations
Pharmaceutical companies must navigate a complex web of regulations that govern electronic records and signatures. In the United States, the FDA’s 21 CFR Part 11 sets the gold standard for electronic records in clinical trials, manufacturing, and distribution, requiring systems to ensure data integrity, audit trails, and non-repudiation. This means any CLM platform must provide verifiable electronic signatures that are legally binding and tamper-proof.
In the European Union, the eIDAS Regulation (electronic IDentification, Authentication and trust Services) establishes a framework for qualified electronic signatures (QES), which carry the same legal weight as handwritten ones. For pharma firms operating across borders, compliance also involves GDPR for data privacy, ensuring that sensitive patient or trial data in contracts isn’t mishandled.
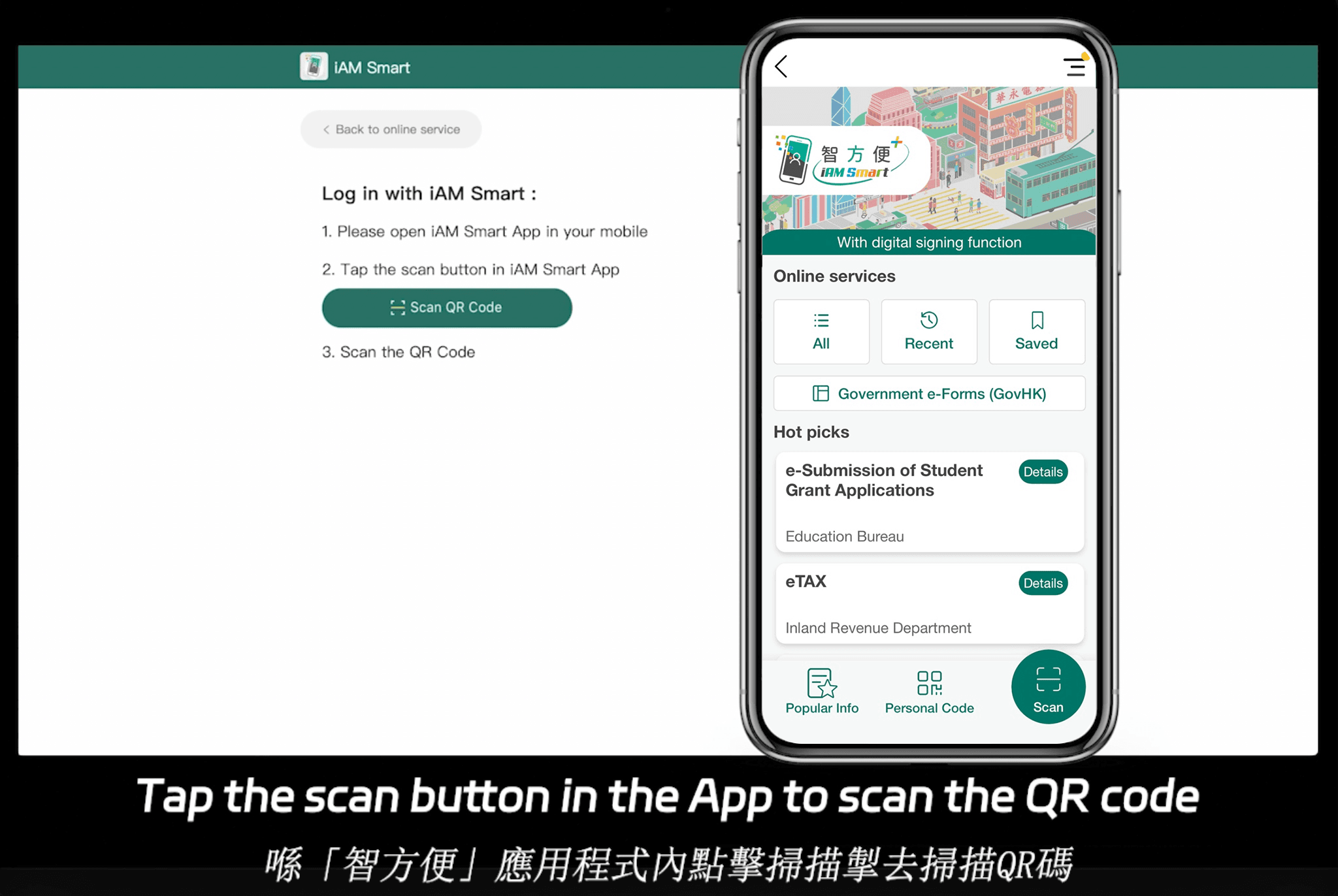
Asia-Pacific regions add further layers of fragmentation. For instance, Singapore’s Electronic Transactions Act aligns with eIDAS but emphasizes ecosystem integration with national digital IDs like Singpass. In Hong Kong, the Electronic Transactions Ordinance requires alignment with tools like iAM Smart for government-to-business (G2B) interactions. China’s regulations, under the Electronic Signature Law, demand high-security protocols for cross-border pharma deals, often involving local data residency. These regional variations highlight why a one-size-fits-all approach falls short; pharma needs tools that adapt to both framework-based standards (like ESIGN in the US) and ecosystem-integrated ones in APAC, where deep API-level docking with government systems is often mandatory.
Challenges in Traditional Contract Management
Manual or legacy systems in pharma expose firms to risks like document forgery, incomplete audit logs, and delays in approvals—critical issues when dealing with intellectual property agreements, clinical trial contracts, or supply chain pacts. A 2023 industry report noted that non-compliance costs the sector over $10 billion annually in fines and rework. CLM solutions address this by automating workflows, enforcing role-based access, and generating immutable records, but only if they meet pharma’s “strict regulatory compliance” bar.
DocuSign CLM: A Tailored Solution for Pharma Compliance
DocuSign’s Contract Lifecycle Management (CLM) platform, integrated with its eSignature core, is designed to tackle these pharma pain points head-on. At its heart is the Intelligent Agreement Management (IAM) suite, which combines AI-driven contract analysis with robust compliance features. For pharmaceutical users, DocuSign CLM ensures end-to-end visibility from drafting to execution and renewal, all while maintaining audit-ready trails.
Core Features for Regulatory Adherence
DocuSign CLM excels in FDA 21 CFR Part 11 compliance through features like electronic signatures with biometric verification, time-stamped audit logs, and encryption for all documents. In clinical trial agreements, for example, the platform’s conditional routing automates approvals based on predefined regulatory clauses, reducing human error. Integration with DocuSign Identify adds advanced authentication, such as knowledge-based or SMS verification, aligning with eIDAS QES requirements for EU pharma exports.
For global operations, DocuSign supports multi-jurisdictional compliance via customizable workflows. In APAC, while it handles basic ESIGN-like standards, users may need add-ons for deeper regional integrations, like SMS delivery for faster signer notifications in high-volume supply contracts. The platform’s analytics dashboard provides real-time compliance reporting, helping pharma legal teams monitor obligations under HIPAA or GDPR.
Pricing for DocuSign CLM starts at enterprise levels, often customized beyond the standard eSignature plans (e.g., Business Pro at $40/user/month annually). Add-ons like Identity Verification incur metered fees, making it scalable for large pharma firms but potentially costlier for smaller biotech players. Overall, DocuSign CLM’s strength lies in its proven track record—serving over 1,000 life sciences clients—with seamless integrations into ERP systems like SAP, crucial for pharma’s complex supply chains.

Comparing Leading CLM and eSignature Platforms for Pharma
To provide a balanced view, here’s a neutral comparison of DocuSign against key competitors: Adobe Sign, eSignGlobal, and HelloSign (now part of Dropbox). This table focuses on pharma-relevant aspects like compliance, pricing, and regional support, based on 2025 public data.
| Feature/Aspect | DocuSign CLM | Adobe Sign | eSignGlobal | HelloSign (Dropbox Sign) |
|---|---|---|---|---|
| Core Compliance (FDA 21 CFR Part 11, eIDAS) | Full support with audit trails, QES, biometric options | Strong eIDAS/QES; FDA via integrations, but less pharma-specific | Global 100+ countries compliant; deep APAC ecosystem (e.g., Singpass, iAM Smart) | Basic ESIGN/eIDAS; limited advanced pharma audits |
| Pharma-Specific Tools | IAM for AI contract review, conditional logic for trials | Workflow automation, but generic; pharma templates available | AI risk assessment, bulk send for HR/supply; regional ID docking | Simple signing; no native CLM or AI features |
| Pricing (Annual, Entry-Level) | $480/user (Business Pro); custom CLM | $10/user/month (Individual); enterprise custom | $199/year (Essential, unlimited users); API included in Pro | $15/user/month; no unlimited seats |
| Regional Strengths | Global, strong in US/EU; APAC add-ons needed | US/EU focus; APAC via partners | APAC optimized (HK/SG data centers); expanding to US/EU | US-centric; basic international |
| Integrations | 400+ (SAP, Salesforce); API plans from $600/year | Adobe ecosystem, Microsoft; API add-on | Lark, WhatsApp; free API in Pro | Dropbox, Google; limited enterprise |
| Limitations for Pharma | Seat-based fees add up; APAC latency in cross-border | Less tailored for fragmented regs; higher customization costs | Newer in US/EU markets; less mature IAM | Lacks deep compliance for global pharma; no bulk/advanced sends |
| Best For | Established pharma with US/EU focus | Creative/digital-heavy pharma teams | APAC-centric firms needing cost-effective regional compliance | Small pharma startups with simple needs |
This comparison underscores that while DocuSign leads in mature markets, alternatives offer niches like cost savings or regional depth.
Adobe Sign: A Versatile Contender in Pharma CLM
Adobe Sign, part of Adobe Document Cloud, provides a solid CLM alternative with emphasis on seamless digital workflows. For pharma, it supports 21 CFR Part 11 through secure e-signatures and detailed reporting, making it suitable for R&D contracts. Its strength is in integrations with Adobe Acrobat for document redaction, aiding HIPAA compliance by anonymizing sensitive data. However, Adobe’s pricing can escalate with enterprise features, starting at $10/user/month for basics but requiring add-ons for advanced pharma audits. While effective in the US and EU, Adobe Sign’s APAC support relies on partnerships, potentially complicating ecosystem-integrated regs like those in Singapore or Hong Kong.

eSignGlobal: Emerging Player with APAC Edge
eSignGlobal positions itself as a compliant eSignature and CLM provider across 100 mainstream global countries, with a particular advantage in the Asia-Pacific region. APAC’s electronic signature landscape is characterized by fragmentation, high standards, and strict regulation—unlike the more framework-based ESIGN/eIDAS models in the US and EU, which rely on email verification or self-declaration. APAC demands “ecosystem-integrated” approaches, involving deep hardware/API-level integrations with government digital identities (G2B), a technical hurdle far exceeding Western norms.
For pharma, eSignGlobal’s AI-Hub offers risk assessment and summarization tailored to regulatory clauses, supporting FDA and eIDAS while excelling in APAC with native ties to Hong Kong’s iAM Smart and Singapore’s Singpass for secure trial enrollments or supply agreements. It’s expanding aggressively into the US and EU to compete directly with DocuSign and Adobe Sign, emphasizing affordability without compromising compliance. The Essential plan, at just $16.6/month ($199/year), allows sending up to 100 documents for electronic signature, unlimited user seats, and verification via access codes—delivering high value on a compliance foundation. For a 30-day free trial, visit their site to test pharma workflows.
HelloSign: Simplicity for Smaller Pharma Operations
HelloSign, rebranded as Dropbox Sign, offers straightforward eSignature for pharma teams needing quick compliance without full CLM complexity. It meets basic ESIGN and eIDAS standards with audit logs, ideal for non-critical contracts like vendor NDAs. Pricing at $15/user/month keeps it accessible, but it lacks advanced pharma features like AI analysis or bulk sends, limiting scalability for global trials.
Strategic Considerations for Pharma Leaders
In evaluating CLM tools, pharma executives should weigh global reach against regional nuances, especially in APAC’s demanding ecosystem. DocuSign CLM remains a benchmark for strict compliance in core markets, but for cost-conscious or APAC-focused operations, alternatives like eSignGlobal provide a neutral, regionally optimized choice as a DocuSign substitute.
Câu hỏi thường gặp

 Chỉ được phép sử dụng email doanh nghiệp
Chỉ được phép sử dụng email doanh nghiệp


